Molecular Medicine and Mechanisms of Disease “M3D” PhD Program
In the M3D PhD program, students receive rigorous interdisciplinary training designed to prepare them for translational research. core courses focus on the mechanisms of disease, the impact of basic science on medicine, and human genetics, providing a rigorous intellectual foundation. Students also participate in one or more courses designed to provide an interface with the clinic and clinical medicine; learn basic statistics; and choose additional electives from the deep and varied menu offered by UW basic science and engineering departments. In addition, students complete three laboratory rotations in their first year with faculty from any of our partner institutions, exploring the innovative science our faculty offers.
The M3D Program is designed for students to complete PhD training in five years, ready to take the next step in diverse careers in academia, biotech, the pharmaceutical industry, education, publishing, or public policy.
The M3D PhD Program is a collaborative effort among the University of Washington School of Medicine, the Department of Laboratory Medicine & Pathology, Seattle Children’s Research Institute, and Fred Hutch Cancer Center.


 M3D Graduates & other Biomedical Hooding Ceremony Honorees – June 2023
M3D Graduates & other Biomedical Hooding Ceremony Honorees – June 2023 M3D Director and Associate Dean of the Graduate School Dr. Bill Mahoney, presenting speech at the 2023 Biomedical Hooding Ceremony
M3D Director and Associate Dean of the Graduate School Dr. Bill Mahoney, presenting speech at the 2023 Biomedical Hooding Ceremony M3D Alumni, Dr. Michael Kiflezghi being hooded by M3D Director Dr. Bill Mahoney at the 2023 Biomedical Hooding Ceremony.
M3D Alumni, Dr. Michael Kiflezghi being hooded by M3D Director Dr. Bill Mahoney at the 2023 Biomedical Hooding Ceremony. M3D Alumna Dr. Miranda Lahman with M3D faculty and mentor, Dr. Aude Chapuis at the 2023 Biomedical hooding Ceremony.
M3D Alumna Dr. Miranda Lahman with M3D faculty and mentor, Dr. Aude Chapuis at the 2023 Biomedical hooding Ceremony. Adair lab at ASGCT 26th Annual Meeting in Los Angeles – May 2023.
Adair lab at ASGCT 26th Annual Meeting in Los Angeles – May 2023. M3D Students at M3D Spring Retreat
M3D Students at M3D Spring Retreat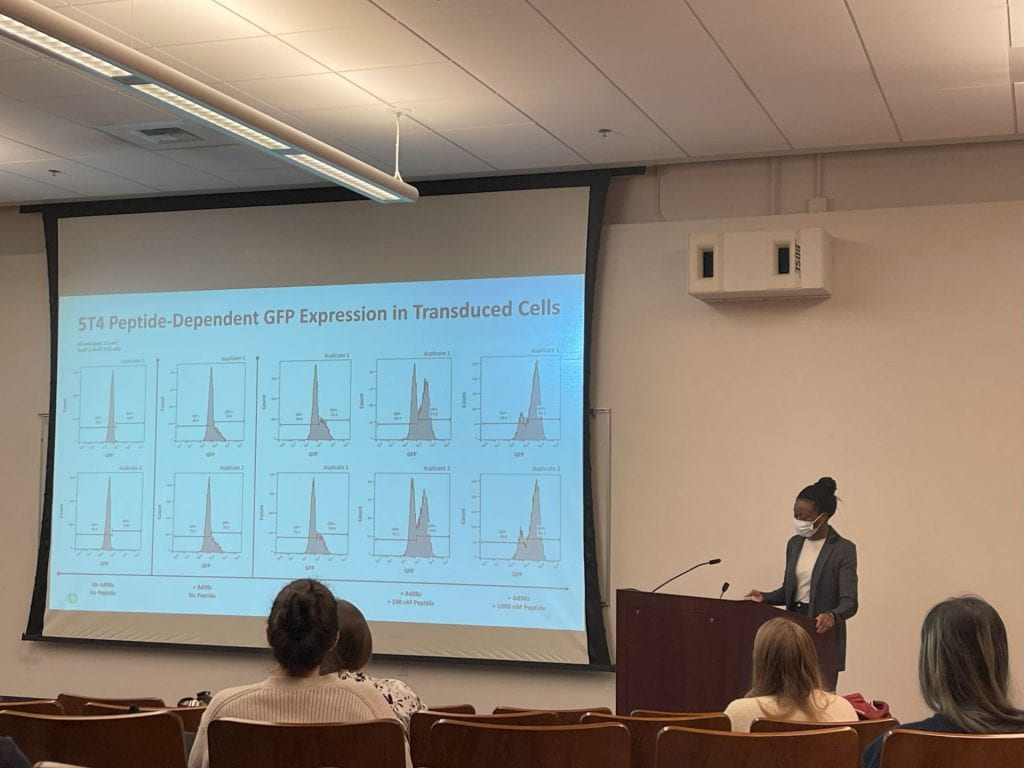 M3D PhD Candidate Iyabode Tiamiyu, presenting a rotation talk.
M3D PhD Candidate Iyabode Tiamiyu, presenting a rotation talk. M3D PhD candidate Cassie Winter presenting poster to M3D PhD candidates Tori Tappen and Jacob Greene at the DLMP Department Retreat.
M3D PhD candidate Cassie Winter presenting poster to M3D PhD candidates Tori Tappen and Jacob Greene at the DLMP Department Retreat.