Murry Laboratory
Clinical and Pathological Aspects of Heart Disease
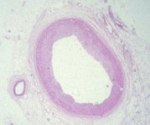
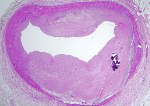
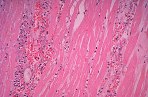
The vast majority of myocardial infarcts result from complications of coronary atherosclerosis. Atherosclerotic plaques can develop surface erosions or rupture their fibrous caps, exposing arterial blood to factors that promote blood thrombosis (clotting). Occlusion of the coronary artery results in myocardial ischemia (deficiency of blood flow to the heart muscle). Myocardium has very high metabolic demands, and once blood flow ceases, irreversible injury to the tissue begins within 20 minutes. Cell death proceeds in a surprisingly orderly fashion over time, beginning in the innermost layers of the ventricular wall (subendocardium) and moving as a wavefront toward the outer layers (subepicardium). If the heart is reperfused (has blood flow restored) by 3 hours, the infarct will be approximately 80% of its final size. If reperfusion is delayed until 6 hours, infarcts are no smaller than those caused by permanent coronary occlusion.
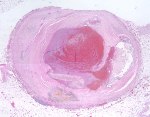
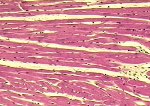
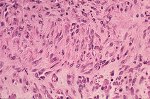
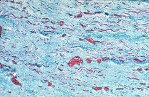
Although there is considerable proliferation of connective tissue and vascular cells after infarction, the heart’s muscle cells, unfortunately, have very little intrinsic regenerative ability. As a result, cardiac muscle that dies from infarction is essentially never replaced. Instead, a wound healing process ensues, where the dead muscle tissue is digested by white blood cells (macrophages), and a provisional repair tissue, termed granulation tissue, grows in to replace the damaged muscle. Granulation tissue (perhaps the only new tissue that adult males can grow), is highly proliferative and rich in blood vessels and connective tissue cells. Granulation tissue remodels over time to form scar tissue. Scar tissue is a tough, fibrous tissue that has good tensile strength, but it is unable to contract. As a result, many patients are left with significant deficits in contractile function after infarction that result in progressive heart failure.
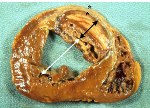
When infarct repair proceeds “well”, the patient is left with a scar, typically smaller than the muscle it replaced, which follows the outline of the original ventricular wall. Often times, however, the infarct wall thins considerably and the ventricular chamber dilates. According to the Law of Laplace, the combination of ventricular chamber dilation and wall thinning greatly increases wall stress, which in turn may contribute to progressive ventricular failure. The constellation of infarct thinning, chamber dilation, muscle cell hypertrophy and ventricular fibrosis are referred to as left ventricular remodeling.